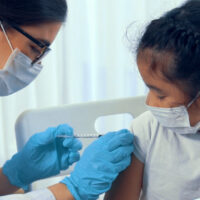
In recent interviews, Health and Human Services Secretary Robert F. Kennedy Jr. has minimized the risk COVID-19 poses to kids and exaggerated the risk of the vaccine, incorrectly claiming that the shot poses a “profound risk” to children. While serious side effects can occur, they are rare, and have not been shown to outweigh the benefits of the vaccine in protecting against COVID-19.
Issues: pediatric vaccination
Injection Protects Babies from RSV Hospitalization, Has Not Been Linked to Deaths
Each year, respiratory syncytial virus hospitalizes 58,000 to 80,000 children under age 5 in the U.S. The Food and Drug Administration recently approved an antibody injection for babies to protect them during the RSV season. There isn’t evidence the shots have killed any babies, contrary to social media claims.
FactChecking Robert F. Kennedy Jr.
Robert F. Kennedy Jr., a prominent anti-vaccine advocate, is running for president as a Democrat. Our SciCheck team has combed through his recent interviews to identify and correct some of his most common health claims in a three-part series. In this first installment, we address several of his talking points about vaccines.
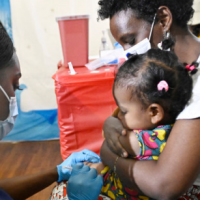
A Guide to COVID-19 Vaccines for the Youngest Kids
Pfizer/BioNTech’s COVID-19 Vaccine Formulation Tweaked to Improve Stability
With the release of its pediatric COVID-19 vaccine, Pfizer switched the buffer used in its formulation to increase the stability of the product, allowing it to remain at refrigerator temperatures for longer. The Food and Drug Administration OK’d the change, which is also being made to some doses for teens and adults. Social media posts, however, misleadingly suggest that the ingredient swap is dangerous or was added to prevent heart attacks in children.