Update, Dec. 20: On Dec. 16, a committee advising the Centers for Disease Control and Prevention voted to recommend the Pfizer/BioNTech and Moderna vaccines over the J&J vaccine because of the risk of a rare but serious blood clotting disorder coupled with low platelets. The CDC endorsed the recommendation the same day. For more information, please see our guide to the J&J vaccine.
Update, May 6, 2022: On May 5, the FDA limited authorized use of the J&J vaccine to adults who either couldn’t get one of the other authorized or approved COVID-19 vaccines because of medical or access reasons, or only wanted a J&J vaccine for protection against the disease. The FDA’s decision was based on an evaluation of the rare risk of TTS and the availability of other vaccines that haven’t shown a risk for the condition. As of April 7, 2022, the CDC and FDA had confirmed 60 cases, including nine deaths, among more than 18.6 million J&J vaccines administered.
So far, nearly 200 million doses of COVID-19 vaccines have been administered in the U.S., including 7.2 million doses of the Johnson & Johnson vaccine. In six cases there have been reports of “a rare and severe type of blood clot” in those who received the J&J vaccine, prompting the Food and Drug Administration and Centers for Disease Control and Prevention to recommend “a pause in the use” of the J&J vaccine “out of an abundance of caution.”
All six cases involved women ages 18 to 48 and their symptoms — which included severe headache, abdominal pain, leg pain or shortness of breath — occurred six to 13 days after they received the J&J vaccine, the agencies said in a joint statement. One died and one remains in critical condition, Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in an April 13 press conference.

The recommended pause only involves the Johnson & Johnson vaccine, which uses a different type of vaccine technology than the other two vaccines that have been authorized in the United States and administered in much greater numbers.
“There have been no red flag signals” from the Pfizer/BioNTech and Moderna vaccines, said Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, in a separate April 13 press conference at the White House.
The six women suffered from a combination of a type of blood clot called cerebral venous sinus thrombosis, or CVST, and low levels of blood platelets, a condition known as thrombocytopenia.
Even though the cases are so few and very rare, Marks said the agencies acted quickly because the usual treatment for blood clots — an anticoagulant drug called heparin — “can actually cause tremendous harm or the outcome can be fatal.”
“So one needs to make sure that providers are aware that if they see people who have low blood platelets, or if they see people who have blood clots, they need to inquire about a history of recent vaccination and then act accordingly in the diagnosis and management of those individuals,” Marks said. “[T]his was taken rapidly in order to honor our commitment to the American public, to ensure that any safety signal that came up during this vaccine rollout was fully addressed in a transparent manner.”
In the press conference, Acting FDA Commissioner Janet Woodcock said the CDC’s Advisory Committee on Immunization Practices will meet on April 14 to further review these cases and assess their potential significance. She said how long the recommended pause lasts depends on what the agencies learn in the next few days. “However, we expect it to be a matter of days,” Woodcock said.

Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and a member of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee, said recommending the pause is a sign the vaccine monitor systems are working and that the agencies discovered and took action on a “very, very rare side effect postapproval.”
“In many ways this should be reassuring to people, in some ways,” Offit said in an interview on CNN. “When this trial was done, the Johnson & Johnson trial in the United States, it involved about 18,000 U.S., 44,000 people worldwide. If this side effect occurs in one in a million people, you’re going to not see that in the preapproval process. But now that it’s out there, it should be reassuring to people to know that people are still looking.”
Update, Dec. 20: As of Dec. 8, the CDC and FDA had confirmed 57 cases of thrombosis with thrombocytopenia syndrome, or TTS, including nine deaths, among more than 16.9 million J&J vaccines administered.
Unlike the Pfizer/BioNTech and Moderna vaccines, which use an mRNA design, the Johnson & Johnson shot uses an adenovirus — a type of virus that typically causes the common cold — modified with the genetic material for SARS-CoV-2 to trigger an immune response.
“With our intensive safety monitoring, we have not detected this type of syndrome with the low platelets among the other vaccines,” Anne Schuchat, principal CDC deputy director, said of the Pfizer/BioNTech and Moderna vaccines. “We have real world evidence now of the vaccines’ effectiveness in the US. We’re taking this pause and precautions around the J&J product in the context of a large, robust, and highly safe and effective vaccination effort.”
A Rare Blood Clot
The rare type of blood clot that the six women experienced is called cerebral venous sinus thrombosis, or CVST, which occurs in the veins in the sinuses that drain blood away from the brain. Johns Hopkins Medicine describes it as a “rare form of stroke.”
“This prevents blood from draining out of the brain,” according to Johns Hopkins.
Schuchat said the risk of CVST is “very low” for those who got the shot more than a month ago. While the cases have been rare, Schuchat said: “For people who recently got the vaccine within the last couple of weeks, they should be aware to look for any symptoms. If you’ve received the vaccine and develop severe headache, abdominal pain, leg pain, or shortness of breath, you should contact your health care provider and seek medical treatment.”
Marks noted that flu-like symptoms were a common side effect in the first few days after vaccination. “So I think for the internist out there and the primary care providers who are caring for patients, if they’re seeing flu-like symptoms and headache for the first few days after a vaccination, that is likely just what has been seen in the common adverse events that are not serious with these vaccines.”
Carlos del Rio, executive associate dean at the Emory School of Medicine & Grady Health System, told CNN the benefits of the Johnson & Johnson vaccine far outweigh the risk for those older than 50 and 18 to 20 years old.
“I want to emphasize the benefit of vaccination far outweigh right now, you know, the risk of dying of COVID,” he said on CNN. “If you are 50 years old — or, you know, if you are 18 or 20 years old, your chance of having this side effect with the J&J vaccine is one in a million. If you were to get COVID, the possibility of you dying from COVID at that age is about 55 in a million.”
CVST occurs annually in 2 to 14 people out of 1 million in the general population, Marks said at the press conference. The concern, however, is that the six cases of CVST — which represent less than 1 in a million of those vaccinated with the J&J vaccine — also had low levels of blood platelets.
“The real thing that is so notable here is not just the cerebral venous sinus thrombosis or the thrombocytopenia. Those two things can occur,” he said. “It’s their occurrence together that makes a pattern. And that pattern is very, very similar to what was seen in Europe with another vaccine. So I think we have to take the time to make sure we understand this complication, and we address it properly.”
Marks was referring to Europe’s experience with the AstraZeneca COVID-19 vaccine — which, like the J&J vaccine, is also an adenovirus-based vaccine. AstraZeneca is not authorized for use in the United States.
AstraZeneca in Europe
Similar to the cases reported in the U.S. with the Johnson & Johnson vaccine, the majority of the cases reported in Europe have occurred in women 60 years old or younger, within two weeks of receiving the AstraZeneca vaccine, according to European Medicines Agency’s safety committee.
The EMA issued a statement on April 7 saying that “unusual blood clots with low blood platelets should be listed as very rare side effects” of the AstraZeneca COVID-19 vaccine, also known as Vaxzevria vaccine.
As of March 22, EMA’s Pharmacovigilance Risk Assessment Committee had reviewed 62 reported cases of blood clots in the brain and 24 reported cases of blood clots in the abdomen, or splanchnic vein thrombosis, in the European Economic Area and the United Kingdom, where 25 million people had received the vaccine. Eighteen of the cases were fatal.
The committee stated that one possible explanation for the combination of blood clots and low blood platelets “is an immune response, leading to a condition similar to one seen sometimes in patients treated with heparin (heparin induced thrombocytopenia, HIT),” a common and potent blood thinner.
Similarly, Marks said at the CDC and FDA press conference that while there is no “definitive cause” for the adverse reaction in the six U.S. cases “the probable cause” may be “an immune response that occurs very, very rarely after some people receive the vaccine, and that immune response leads to activation of the platelets, and these extremely rare blood clots.”
According to an April 11 news report in Science, at least 222 suspected cases have been reported in Europe after 34 million individuals have received their first dose of the vaccine, and more than 30 have died.
A team of scientists led by clotting expert Andreas Greinacher of the University of Greifswald has been studying 11 cases of what he’s calling “vaccine-induced immune thrombotic thrombocytopenia” in Germany and Austria. A second team is looking at five cases in Norway. Both likened the condition to what doctors see in patients treated with heparin, according to a news report by STAT.
The EMA concluded that given the associated risks of COVID-19 and the fact that the reported combination of blood clots and low blood platelets “is very rare,” “the overall benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects.” But according to Science, many countries are restricting the use of the AstraZeneca vaccine for younger people who have less risk of getting severely ill from COVID-19. Germany is using the vaccine only in people 60 years old or older, France in those 55 years old or older, and the United Kingdom is recommending that those younger than 30 years old get another vaccine.
On April 12, Johnson & Johnson began delivering doses of its COVID-19 vaccine to countries in Europe. But a day later, Johnson & Johnson said in a statement that it was reviewing the U.S. cases with health authorities in Europe and had “made the decision to proactively delay the rollout of our vaccine in Europe.”
“We have been working closely with medical experts and health authorities, and we strongly support the open communication of this information to healthcare professionals and the public,” the statement said.
Johnson & Johnson Clinical Trials
The FDA issued Janssen Biotech Inc., a Johnson & Johnson pharmaceutical company, an emergency use authorization for the vaccine on Feb. 27.
On CNN, Offit was asked why this adverse reaction involving blood clots did not show up in the J&J clinical trials.
“Because it was too rare,” Offit said. “If you look at the way the J&J did their trial, that was roughly a 44,000-person trial, 18,000 U.S. If an event is occurring in one in a million people, you’re unlikely to see that. But again, I just want to make the point that it should be reassuring to people that we’re always looking, always looking to make sure that there isn’t even a very, very rare side effect, and that should be reassuring to people.”
As we have reported, there were 44,325 participants in J&J’s phase 3 trial — 21,895 received the COVID-19 vaccine and 21,888 were in the placebo group.
The FDA determined three non-fatal serious adverse events in the vaccinated group were likely related to the vaccine: a hypersensitivity reaction involving urticaria or hives, injection site pain unresponsive to over-the-counter pain medicine, and a case involving a few days of symptoms including “generalized malaise, weakness, myalgia, shortness of breath, headache, sensation of numbness and tingling in upper extremities, chest pain and fever.”
The safety “analysis supported a favorable safety profile with no specific safety concerns identified that would preclude issuance of an EUA,” the FDA briefing document said.
There was one reported case of a 25-year-old male during the trial who experienced a transverse sinus thrombosis 21 days after receiving the J&J vaccine. The event caused a pause in the study trial, but ultimately was ruled unrelated to the vaccine.
“After thorough investigation and expert consultation no clear cause of the event was identified; however possible contributing factors, such as preceding infection and an anatomical anomaly, were suggested,” the FDA briefing document said. “The investigator’s brochure and informed consent form were updated accordingly, and the study pause was lifted. The investigator and Sponsor’s final assessment of this event was that it was not related to the study product.”
Impact on U.S. Vaccination Goals
The CDC and FDA did not order a halt to administering the J&J vaccine, and the pause is not expected to last long, as we noted earlier.
“This is a recommendation and it’s not a mandate,” Marks said. “It’s out of an abundance of caution. We’re recommending that the vaccine be paused in terms of its administration. However, if an individual health care provider has a conversation with an individual patient and they determined that the benefit-risk for that individual patient is appropriate, we’re not going to stop that provider from administering the vaccine, because it could be right in many cases, that benefit-risk will be beneficial overall to that individual in the large majority of cases.”
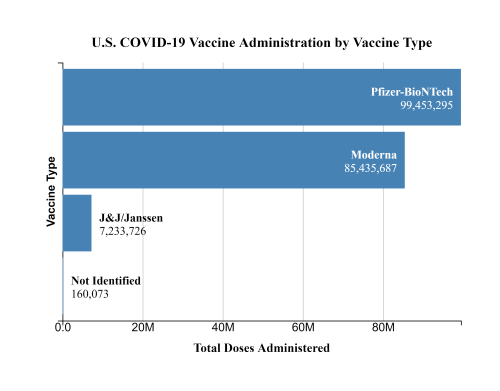
The recommended pause also should not disrupt President Joe Biden’s goal to have 200 million doses administered in his first 100 days. Jeff Zients, the White House COVID-19 response coordinator, said Johnson & Johnson’s vaccine makes up less than 5% of the shots administered in the U.S. so far.
“[T]he United States has secured enough Pfizer and Moderna doses for 300 million Americans,” Zients said. “This is more than enough supply to continue the current pace of vaccinations of 3 million shots per day, and meet the President’s goal of 200 million shots by his 100th day in office — and continue on to reach every adult who wants to be vaccinated.”
On CNN, del Rio praised the CDC and FDA for acting quickly and making vaccine safety a priority. He expressed concern, however, about the potential for the recommended pause to increase vaccine hesitancy — despite the overwhelmingly evidence that the benefits of COVID-19 vaccines far outweigh the rare risks.
“These vaccines have saved thousands of lives already,” he said. “People have — we have seen mortality in the U.S. continue to decline despite cases going up and that’s because we’re vaccinating people. … I would still recommend people to get vaccinated. I would not say do not get vaccinated just because of this very rare side effect.”
For more information, see “Q&A on the Rare Clotting Events That Caused the J&J Pause.”
Editor’s note: SciCheck’s COVID-19/Vaccination Project is made possible by a grant from the Robert Wood Johnson Foundation. The foundation has no control over our editorial decisions, and the views expressed in our articles do not necessarily reflect the views of the foundation. The goal of the project is to increase exposure to accurate information about COVID-19 and vaccines, while decreasing the impact of misinformation.