The COVID-19 vaccines currently in use must be administered via injection. But Instagram posts baselessly suggest that Bill Gates and George Soros will use COVID-19 tests to secretly vaccinate people who haven’t yet received the shots. There is no evidence for that conspiracy theory, and scientists say trying to administer a vaccine with a swab would likely not be effective.
Misconception: Vaccination
Video Twists Advice on Delta Variant and Vaccination
An epidemiologist recommended that people get the COVID-19 vaccine because some evidence suggests an unvaccinated person who gets the delta variant is “twice as likely to require hospital treatment” than someone infected with the alpha variant. But a Facebook video twists that advice to claim that he said vaccinated people would be twice as likely to be hospitalized.
Viral Posts Lift Bogus ‘Quarantine’ Story from Satire Site
Mayim Bialik and Sons Got COVID-19 Vaccine
Photo Shows 2018 France World Cup Celebration, Not Vaccine Protest
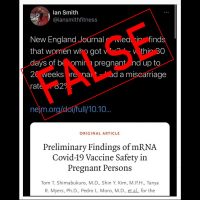
CDC Data Thus Far Show COVID-19 Vaccination Safe During Pregnancy
Federal vaccine monitoring systems have identified no safety concerns with the COVID-19 vaccines for pregnant people. Preliminary Centers for Disease Control and Prevention data show that miscarriage is not more frequent than expected in vaccinated people. Online posts, however, falsely contend that such data, as reported in a CDC publication, show an 82% miscarriage rate.
Meme Spreads Falsehood About Vaccine Transfer Through Eating Meat
Baseless Conspiracy Theory Follows Deaths of Haitian President, Other National Leaders
Following the assassination of Haitian President Jovenel Moïse, social media posts baselessly suggest that he and other world leaders were killed or died because they opposed COVID-19 vaccination in their countries. All the leaders named in the posts, except Moïse, died of natural causes. At least one supported vaccination.
Greene’s Deceptive Claims of Forced COVID-19 Vaccinations and Vaccination ‘Deaths’
There is no evidence that a door-to-door campaign to encourage vaccinations against COVID-19 means President Joe Biden and Democrats “are coming to your front door to force you to take the vax,” as Rep. Marjorie Taylor Greene tweeted. She also cited a figure for reported deaths after vaccination, which is not the same as deaths caused by vaccination.
Spoof Video Furthers Microchip Conspiracy Theory
A list of the ingredients used in COVID-19 vaccines is publicly available, and the ingredients don’t include microchips. Yet claims advancing conspiracy theories that they do continue to flourish. A recent video purports to show a microchip reader for pets detecting a chip in a vaccinated person’s arm — but the original video was created as a joke.